This is the only rational response a person can make after they are presented with irreducible complexity.
"To get a protein we need DNA first. But, to even have DNA we need many different DNA repair systems.
Besides DNA repair, we also need a way to copy DNA. Without the faithful passing on of uncorrupted information from one generation to the next, life would be impossible.
Dozens of proteins and RNAs are required to duplicate DNA. These include helicases that open the strands, topoisomerases that keep the strands from getting kinked, and single-strand binding proteins that help to hold the strands open.
Meanwhile, two different DNA polymerases are used (one for each strand). On one strand, DNA copying is simple and direct. On the other strand, however, it is performed in short segments (Okazaki fragments) that must be joined with DNA ligase after DNA primase adds a short RNA primer to the strand. Confused yet? The point here is to show how many complex systems must already be in place before we can start making our desired protein.
The next step in our chain of spaghettification deals with cell signalling and the control of information flow. I don’t know how it knows what it needs, but let’s say the cell decides it needs more ATP synthase motors. The genes for the necessary proteins are buried somewhere in the genome, but the genome is a giant, four-dimensional ball of thin, sticky, fragile strings. Somewhere in that ball of string is our target gene. If it is not currently active, it might be tucked away and so must be found. It also might be deactivated and so must be turned on. The DNA must also be unwound at that specific location.
There are many different aspects of DNA storage within the cell. First, DNA is wrapped around histone proteins. These histones have a long tail that can be chemically modified. Thus, the cell can ‘tag’ DNA in different ways. The DNA itself can also be tagged. For example, by adding methyl groups to it, a strand of DNA is effectively turned off.
What I am describing is something called epigenetics. This is the information layer that is added to DNA and the associated histones that tells the cell which areas of the genome are active, etc. Guess what? Epigenetic processes require many additional proteins that must all be maintained in the DNA and that must all be manufactured. Without epigenetics, the cell can’t regulate the genome and so it will never be able to manufacture the desired protein, but epigenetics burns even more ATP.
Now that all those systems are in place, the cell is finally ready to start making the protein! First, the cell will make a copy of the protein-coding gene in a process called transcription.
The next step in our chain of spaghettification deals with cell signalling and the control of information flow. I don’t know how it knows what it needs, but let’s say the cell decides it needs more ATP synthase motors. The genes for the necessary proteins are buried somewhere in the genome, but the genome is a giant, four-dimensional ball of thin, sticky, fragile strings. Somewhere in that ball of string is our target gene. If it is not currently active, it might be tucked away and so must be found. It also might be deactivated and so must be turned on. The DNA must also be unwound at that specific location.
There are many different aspects of DNA storage within the cell. First, DNA is wrapped around histone proteins. These histones have a long tail that can be chemically modified. Thus, the cell can ‘tag’ DNA in different ways. The DNA itself can also be tagged. For example, by adding methyl groups to it, a strand of DNA is effectively turned off.
What I am describing is something called epigenetics. This is the information layer that is added to DNA and the associated histones that tells the cell which areas of the genome are active, etc. Guess what? Epigenetic processes require many additional proteins that must all be maintained in the DNA and that must all be manufactured. Without epigenetics, the cell can’t regulate the genome and so it will never be able to manufacture the desired protein, but epigenetics burns even more ATP.
Now that all those systems are in place, the cell is finally ready to start making the protein! First, the cell will make a copy of the protein-coding gene in a process called transcription.
The copy will be in the form of RNA, not DNA. They use a slightly different sugar (ribose vs. deoxyribose). This makes RNA 100× less stable than DNA, which is really saying something! But the main difference between these two molecules is that RNA has uracil (U) instead of thymine (T). For transcription to occur, the DNA must be unwound and the histones must be removed. Similar to DNA replication, a flurry of accessory proteins are involved.
Not only does the creation of all these proteins use up a lot of ATP, but the ‘letters’ in DNA and RNA are also dependent on ATP. For example, ATP is adenosine triphosphate. This is also the source for the A (adenine) in DNA and RNA. The other letters (G, C, T, and U) are manufactured from CTP, GTP, TTP, and UTP, respectively. The biochemical pathways are complex, and each step in the manufacturing chain is driven by proteins (which require ATP to be produced, of course), but the creation of CTP, GTP, TTP, and UTP require the burning of even more ATP.
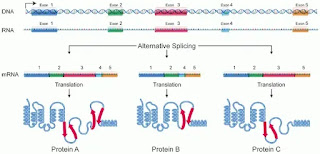
The intron-exon break points generally contain 12–19 short ‘motifs’ that tell the cell where to cut and how to join the exons together.
Exons can be alternately joined to create many different proteins from a single ‘gene’. This allows the cells to produce several hundred thousand unique proteins from less than 20,000 genes. Thus, our ATP synthase protein might be an alternative product, one protein among a family of similar proteins that are all coded by the same gene. This splicing and dicing requires more proteins and burns up even more ATP, but now we have an RNA that can be used to manufacture our protein.
The manufacturing of a raw protein is nothing short of an engineering miracle. The cell starts with a complex machine called a ribosome. This is composed of multiple proteins and RNAs, each of which must be coded in the DNA, manufactured with ATP-dependent processes … you get the drill.
The mRNA is fed into the ribosome from one end. The mRNA will be translated into protein three letters at a time. Each set of three letters is called a ‘codon’. There are 64 possible codons and 21 amino acids, so some amino acids are ‘coded’ by more than one codon. Three of the codons are used as ‘stop’ signals, but one of them can also code for the amino acid selenocysteine.
A series of adaptor molecules called transfer RNAs (tRNA) enter the ribosome. At the base of each tRNA is a three-letter ‘anti-codon’ that matches a codon. At the top of each tRNA is an amino acid that is
removed from the tRNA and added to the growing protein strand. Each tRNA is ‘charged’ with its amino acid by a specific protein in the aminoacyl transferase family. There are elaborate mechanisms to make sure the right amino acid is charged, not the wrong one, even if chemically similar. Not only do these proteins require ATP in their manufacture, but the charging step and the protein elongation stepalso burn ATP.
Most proteins, if left to themselves, will fold up into a useless knot of random coils. Thus, they need help folding. After the strand leaves the ribosome, helper proteins called chaperones clamp onto the unfolded strand. The protein is then escorted to, and inserted into, a huge multi-protein molecule called a chaperonin. We’re not exactly certain what happens inside, but the chaperonin will fold the protein into its near-finished state and spit it out the other end. More proteins. More activity. More ATP burned.
The final stage in the manufacturing process is delivery. Proteins must be actively transported to the site where they are needed. To do that, the cell uses a kinesin protein. There are many different kinesin types, but the main one we need to know about is a slender molecule with two legs. Those legs literally walk along a microtubule (made of proteins that require ATP in their manufacturing, transport, and assembly). Each step requires one ATP. The package to be delivered is stored on the top end of the kinesin and various signals and modifications to it effectively serve as a postal address."
Not only does the creation of all these proteins use up a lot of ATP, but the ‘letters’ in DNA and RNA are also dependent on ATP. For example, ATP is adenosine triphosphate. This is also the source for the A (adenine) in DNA and RNA. The other letters (G, C, T, and U) are manufactured from CTP, GTP, TTP, and UTP, respectively. The biochemical pathways are complex, and each step in the manufacturing chain is driven by proteins (which require ATP to be produced, of course), but the creation of CTP, GTP, TTP, and UTP require the burning of even more ATP.
The intron-exon break points generally contain 12–19 short ‘motifs’ that tell the cell where to cut and how to join the exons together.
Exons can be alternately joined to create many different proteins from a single ‘gene’. This allows the cells to produce several hundred thousand unique proteins from less than 20,000 genes. Thus, our ATP synthase protein might be an alternative product, one protein among a family of similar proteins that are all coded by the same gene. This splicing and dicing requires more proteins and burns up even more ATP, but now we have an RNA that can be used to manufacture our protein.
The manufacturing of a raw protein is nothing short of an engineering miracle. The cell starts with a complex machine called a ribosome. This is composed of multiple proteins and RNAs, each of which must be coded in the DNA, manufactured with ATP-dependent processes … you get the drill.
The mRNA is fed into the ribosome from one end. The mRNA will be translated into protein three letters at a time. Each set of three letters is called a ‘codon’. There are 64 possible codons and 21 amino acids, so some amino acids are ‘coded’ by more than one codon. Three of the codons are used as ‘stop’ signals, but one of them can also code for the amino acid selenocysteine.
A series of adaptor molecules called transfer RNAs (tRNA) enter the ribosome. At the base of each tRNA is a three-letter ‘anti-codon’ that matches a codon. At the top of each tRNA is an amino acid that is
removed from the tRNA and added to the growing protein strand. Each tRNA is ‘charged’ with its amino acid by a specific protein in the aminoacyl transferase family. There are elaborate mechanisms to make sure the right amino acid is charged, not the wrong one, even if chemically similar. Not only do these proteins require ATP in their manufacture, but the charging step and the protein elongation stepalso burn ATP.
Most proteins, if left to themselves, will fold up into a useless knot of random coils. Thus, they need help folding. After the strand leaves the ribosome, helper proteins called chaperones clamp onto the unfolded strand. The protein is then escorted to, and inserted into, a huge multi-protein molecule called a chaperonin. We’re not exactly certain what happens inside, but the chaperonin will fold the protein into its near-finished state and spit it out the other end. More proteins. More activity. More ATP burned.
The final stage in the manufacturing process is delivery. Proteins must be actively transported to the site where they are needed. To do that, the cell uses a kinesin protein. There are many different kinesin types, but the main one we need to know about is a slender molecule with two legs. Those legs literally walk along a microtubule (made of proteins that require ATP in their manufacturing, transport, and assembly). Each step requires one ATP. The package to be delivered is stored on the top end of the kinesin and various signals and modifications to it effectively serve as a postal address."
CMI